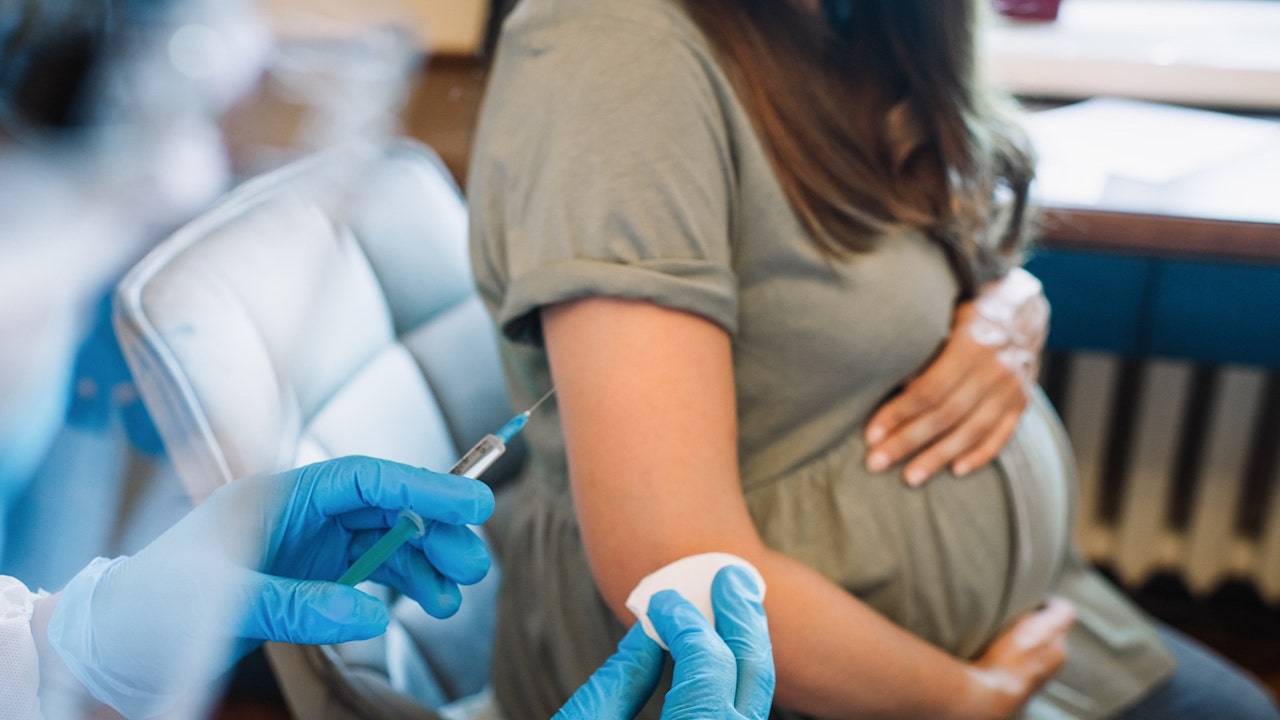
The Food and Drug Administration (FDA) made an exciting announcement on Monday, approving the first-ever vaccine for pregnant women to prevent RSV in infants. This milestone approval of Abrysvo as a maternal vaccine to prevent lower respiratory tract disease (LRTD) in babies from birth through 6 months of age was highlighted in the FDA’s press release.
The vaccine is administered via injection between 32 and 36 weeks of gestation and is a single-dose vaccine. This approval for pregnant women follows the FDA’s previous announcement in May, approving Abrysvo to prevent LRTD in individuals aged 60 and older.
RSV, or respiratory syncytial virus, is a common infection that affects the lungs and respiratory tract. It is especially prevalent among children and most individuals are infected before the age of 2. However, high-risk groups including infants under 12 months, older adults, and those with heart and lung disease or weak immune systems are more susceptible to serious illness, as stated by the Mayo Clinic.
The FDA recognizes RSV as the leading cause of lower respiratory tract illness worldwide, and it is the primary reason for infant hospitalizations in the United States. Therefore, the approval of Abrysvo as the first and only maternal immunization to protect newborns from RSV from birth through six months is a significant achievement in the scientific and public health communities, as expressed by Annaliesa Anderson, PhD, Senior Vice President and Chief Scientific Officer at Pfizer.
Dr. Peter Marks, Director of the FDA’s Center for Biologics Evaluation and Research, also acknowledged the severity of RSV in children and the importance of protecting infants from this life-threatening disease. He commended Abrysvo’s approval, which will provide healthcare providers and pregnant individuals with an option to safeguard infants.
The approval of Abrysvo by the FDA follows rigorous clinical studies that examined the vaccine’s efficacy, safety, and immunogenicity against LRTD and severe LRTD due to RSV in infants born to vaccinated pregnant individuals. Pfizer reported the positive results of these studies in The New England Journal of Medicine in April 2023.
Among the 3,500 pregnant women who received the vaccine, there was an impressive over 81% reduced risk of their infants developing severe lower respiratory tract disease within the first three months of birth compared to those who received placebos.
It’s worth mentioning that pregnant women who received Abrysvo experienced some common side effects like muscle pain, headache, nausea, and pain at the injection site. Additionally, there was a slightly higher incidence of preeclampsia, low birth weight, preterm birth, and jaundice in infants born to mothers who received Abrysvo compared to the placebo group.
Melissa Rudy, the health editor and a member of the lifestyle team at Fox News Digital, provided this informative content.
Denial of responsibility! VigourTimes is an automatic aggregator of Global media. In each content, the hyperlink to the primary source is specified. All trademarks belong to their rightful owners, and all materials to their authors. For any complaint, please reach us at – [email protected]. We will take necessary action within 24 hours.