Scientists Develop New Tool for Determining the Age of Eye Cells
A team of researchers from Stanford University has successfully developed a groundbreaking tool that allows for the age determination of eye cells without the need for regenerative tissue sampling. This innovative technique has the potential to revolutionize the field of eye disease treatment by making it more personalized and targeted.
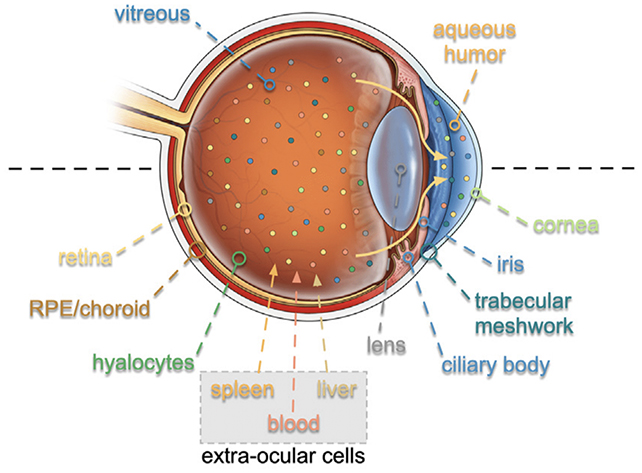
By adapting a method used for analyzing eye fluid, the scientists were able to detect nearly 6,000 proteins present in the fluid that could be traced back to the cells of the eye. Among these proteins, 26 were found to be collectively associated with eye aging.
The breakthrough was achieved through the utilization of an artificial intelligence system that was trained on the eye fluid samples of 46 healthy patients. By cross-referencing the patients’ ages with the detected proteins, the AI system was able to accurately predict an individual’s age based on their eye fluid.
“Understanding the molecules is the first crucial step in the development of any successful therapy,” says ophthalmologist Vinit Mahajan from Stanford University, emphasizing the significance of this breakthrough.
Furthermore, after establishing their eye-aging clock, the researchers investigated how specific eye diseases could accelerate the aging of cells. They conducted further analysis on eye fluid samples obtained from 62 patients with various eye diseases.
The results revealed that certain proteins exhibited higher levels of aging in patients with early-stage diabetic retinopathy (retinal cell damage due to reduced blood supply) and uveitis (inflammation inside the eye). These proteins indicated that such patients could be predicted to be 12 years and nearly 30 years older, respectively, compared to healthy individuals.
Using a novel approach called TEMPO (tracing expression of multiple protein origins), the team traced the affected proteins back to the RNA (ribonucleic acid) responsible for their creation. This allowed them to identify the problematic cells causing these diseases, which were found to differ for each respective condition.
“Patients with the same disease may present different manifestations at the molecular level,” explains Mahajan. “With the molecular fingerprint we’ve developed, we can identify drugs that are effective for each individual patient.”
This breakthrough addresses a longstanding challenge encountered in studying diseases in the eye, as well as other areas like the brain. By eliminating the need for tissue samples, which can cause significant damage and hinder cell regeneration, this technique opens up new possibilities for research and treatment.
The researchers also speculate that the methodology they have demonstrated here could be applied to other bodily fluids, providing experts with invaluable biomarkers for disease treatment and potentially prevention.
“It’s as if we’re holding these living cells in our hands and examining them with a magnifying glass,” remarks Mahajan. “We’re gaining a microscopic understanding of our patients, enabling precision health and more informed clinical trials.”
The research has been published in the prestigious scientific journal Cell.


