An Array Revolutionizing Neuroscience
Source: Precision Neuroscience
The moment was fleeting, and Craig Mermel almost missed it.
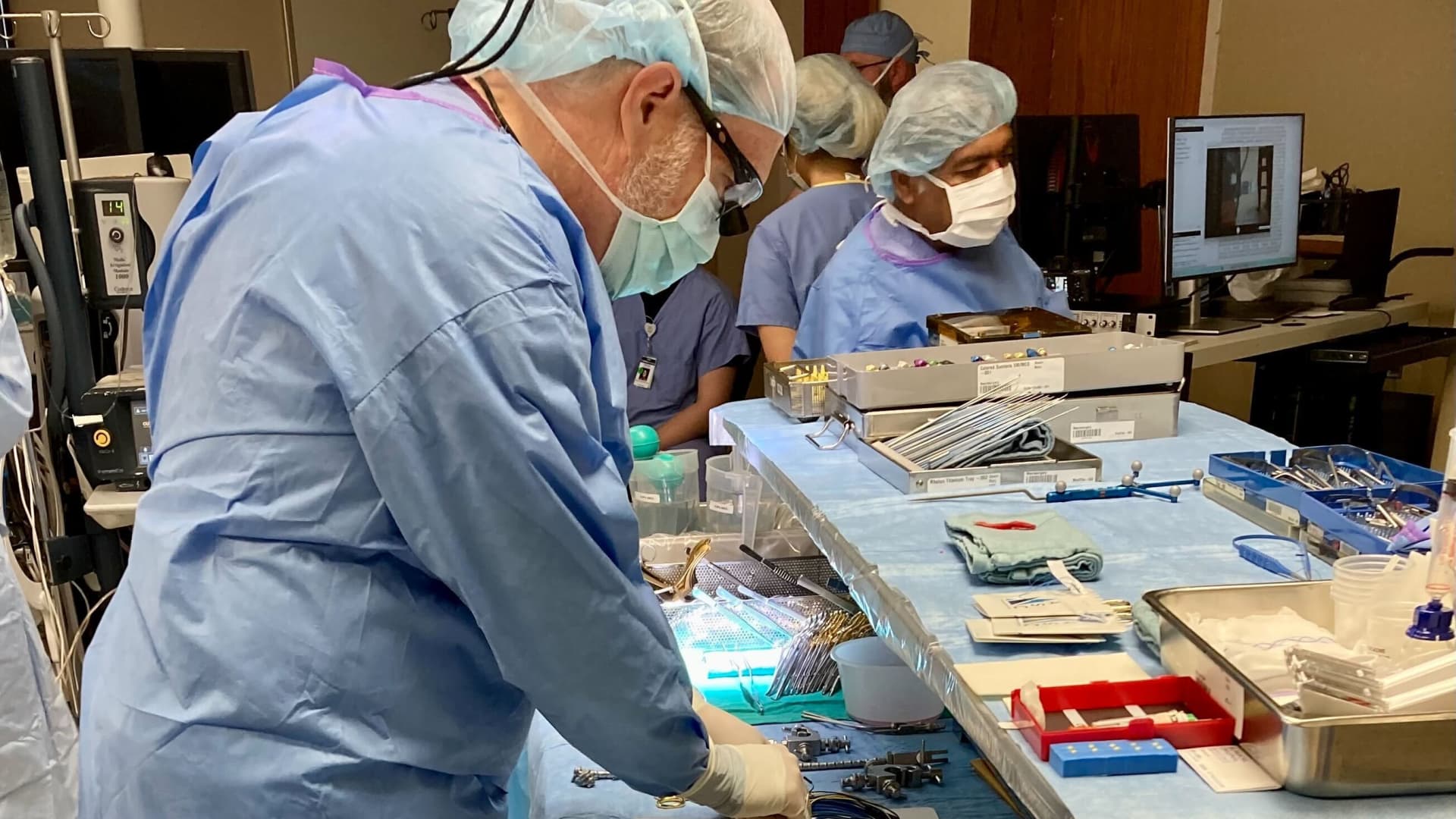
He found himself in a bustling operating room in West Virginia, eagerly awaiting the placement of Precision Neuroscience’s neural implant system onto a conscious patient’s brain. Mermel, the president and chief product officer of Precision, momentarily glanced away, and when he turned back, the company’s ultra-thin electrode array was already in position.
Within seconds, a high-resolution depiction of the patient’s brain activity appeared on a screen, capturing the most detailed image of human thought ever recorded, according to Precision. Mermel described the experience as almost surreal, admitting to feeling chills at the incredible nature of the data and its visualization.
The procedure witnessed by Mermel marked the first-ever in-human clinical study conducted by the company.
Precision Neuroscience, established in 2021 by one of the co-founders of Neuralink, Elon Musk’s brain-computer interface startup, is a leading competitor in the field. Their mission is to aid patients with paralysis in operating digital devices by decoding their neural signals. Other companies such as Synchron, Paradromics, and Blackrock Neurotech have also developed devices with similar capabilities. In January, Precision announced a successful Series B funding round, securing $41 million in investments.
At the core of the company’s offerings is their flagship BCI system, the Layer 7 Cortical Interface. Resembling a strip of scotch tape, Precision claims that this electrode array, thinner than a human hair, can delicately conform to the brain’s surface without causing any damage. In the clinical study, Precision temporarily placed the system on the brains of three patients who were undergoing tumor removal neurosurgery.
Having achieved successful results in this initial trial, Precision plans to expand their research to explore further applications in clinical and behavioral contexts. According to Mermel, if these trials prove fruitful, patients with conditions like ALS could regain some ability to communicate and interact using their thoughts, such as controlling cursors, typing, and accessing social media.
However, the path to market for this technology remains long and arduous, despite this significant milestone. Precision has yet to receive FDA approval for their device, and they will need to work closely with regulators to conduct rigorous testing and ensure data safety. Notably, as of June, no BCI company has obtained final FDA clearance.
“Our objective is to develop a device that can aid individuals with permanent disabilities, so this is just the first step,” explained Mermel. “Now, the real work begins.”
Doctors preparing Precision’s revolutionary system. Precision’s array compared to a penny.
Photo: Anna von Scheling
According to Dr. Benjamin Rapoport, co-founder and chief science officer at Precision, numerous academic medical centers expressed their interest in supporting the company’s pilot clinical study. Ultimately, Precision partnered with West Virginia University’s Rockefeller Neuroscience Institute, preparing for the procedures meticulously for over a year.
Having worked on BCI technology for more than two decades, Rapoport described the moment when Precision’s technology was applied to a human patient’s brain as an “incredibly gratifying” milestone.
Dr. Peter Konrad, chairman of the Department of Neurosurgery at the Rockefeller Neuroscience Institute, personally placed Precision’s system onto the patients’ brains during the procedures. He likened the process to placing a delicate piece of tissue paper onto the brain’s surface.
The patients had the Layer 7 system on their brains for 15 minutes. One patient remained asleep, while two others were woken up to enable the capture of their brain activity as they spoke.
“I’ve never witnessed such an extensive amount of data, 1,000 channels of electrical activity in real-time, washing over the brain as someone was talking,” Konrad stated in an interview with CNBC. “It was as if we were observing the process of thought. Truly remarkable.”
While electrodes are commonly used during neurosurgery to monitor brain activity, the resolution offered by conventional systems is limited. Konrad explained that standard electrodes are approximately four millimeters in size, whereas Precision’s array can have 500 to 1,000 contacts within that same area.
“It’s akin to the difference between viewing the world through an old black and white camera versus experiencing stunning high-definition imagery,” he added.
For the patients in this study, it is too early to experience the direct benefits of this groundbreaking technology.
Precision’s array compared to a penny.
Photo: Anna von Scheling
Precision envisions a future where their technology eliminates the need for open brain surgery altogether. In an interview with CNBC in January, co-founder and CEO Michael Mager suggested that surgeons should be able to implant the array by making a small incision in the skull and sliding the device in, much like inserting a letter into a mailbox. This incision would be less than a millimeter thick, making it unnecessary to shave the patient’s hair for the procedure.
This minimally invasive approach adopted by Precision stands in contrast to other BCI companies such as Paradromics and Neuralink, which design systems intended for direct insertion into brain tissue.
Rapoport explained that while inserting a BCI directly into the brain would provide a clearer understanding of each neuron’s function, it risks damaging the tissue and poses challenges for scalability. He emphasized that Precision’s goals of decoding speech and achieving other desired functionalities do not require such detailed insights, and thus, the company made this tradeoff.
In the following weeks, Precision plans to replicate the procedure with two additional patients as part of their pilot clinical study. Rapoport mentioned that the company has already submitted their initial findings to a scientific journal, and making the data publicly available will mark a significant next step.
Precision also has similar studies underway with renowned health systems such as Mount Sinai in New York City and Massachusetts General Hospital in Boston. Rapoport expressed his hope that Precision will receive full FDA clearance for their first-generation device by next year.
“Witnessing the early results firsthand is truly gratifying,” Rapoport shared. “If you’re fortunate, there are a few moments in your life when you get to see something before anyone else in the world.”
Denial of responsibility! VigourTimes is an automatic aggregator of Global media. In each content, the hyperlink to the primary source is specified. All trademarks belong to their rightful owners, and all materials to their authors. For any complaint, please reach us at – [email protected]. We will take necessary action within 24 hours.