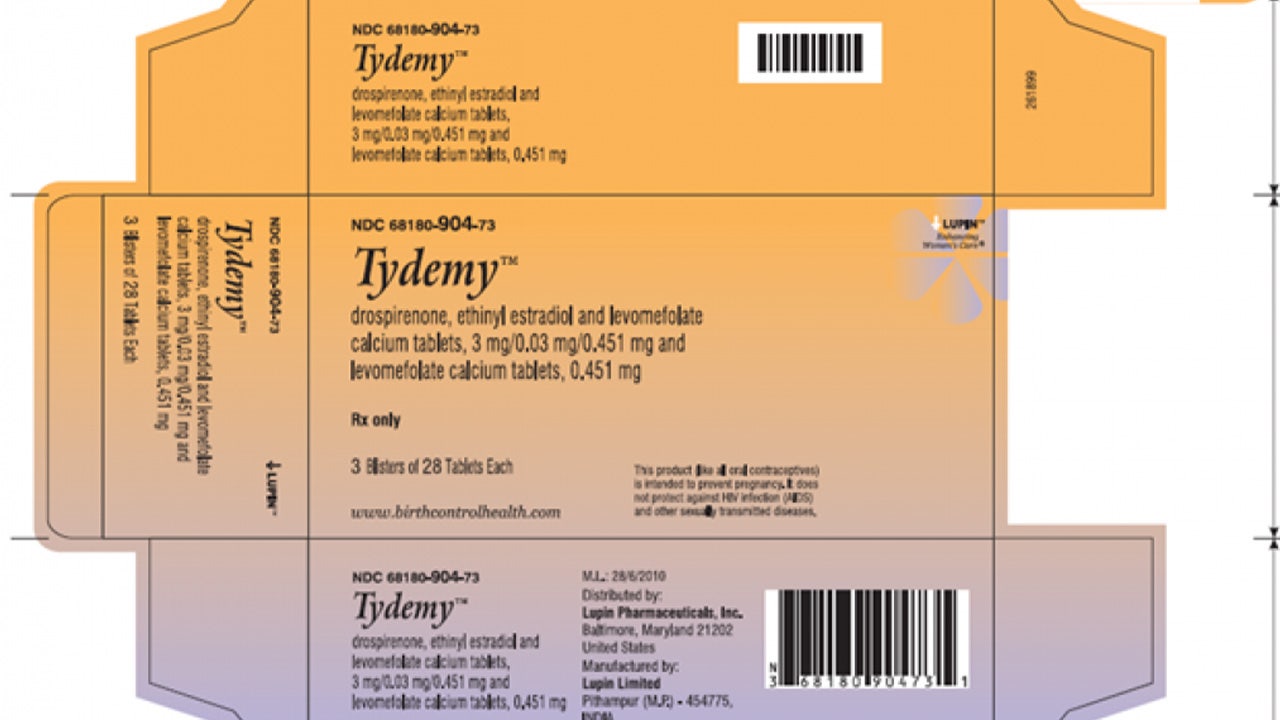
Lupin Pharmaceuticals, a renowned pharmaceutical company, has issued a voluntary recall for two batches of their oral birth control product, Tydemy. The recall comes as a result of reduced effectiveness in the product, which could potentially lead to unexpected pregnancies among its users. The Food and Drug Administration (FDA) has reported that the recall was initiated after the manufacturers discovered that the oral contraception contained lower levels of ascorbic acid, an essential ingredient, and higher levels of a “known impurity.”
The company has expressed concerns that the decreased levels of ascorbic acid may impact the product’s efficacy, potentially resulting in unexpected pregnancies. However, it’s important to note that the FDA has not received any reports of adverse events related to the use of this product.
The recalled Tydemy pills are a combination prescription contraceptive containing estrogen and progestin. The affected batches, identified as lots L200183 and L201560, were distributed by pharmacies throughout the United States from June 3, 2022, to May 31, 2023. Consumers can find the lot number on the side of the label.
The FDA has confirmed that a total of 4,179 boxes of the oral birth control have been recalled. Individuals who are currently taking the recalled lots are advised to continue taking their pills but should immediately consult their doctor to seek an alternative.
This is not the first voluntary recall by Lupin Pharmaceuticals. In December of the previous year, they recalled one batch of 20-milligram Quinapril Tablets USP and three batches of 40-milligram Quinapril Tablets USP due to the potential presence of a nitrosamine impurity.
For more health-related news, sign up for our newsletter by clicking here.
(Source: Fox News)
Denial of responsibility! VigourTimes is an automatic aggregator of Global media. In each content, the hyperlink to the primary source is specified. All trademarks belong to their rightful owners, and all materials to their authors. For any complaint, please reach us at – [email protected]. We will take necessary action within 24 hours.