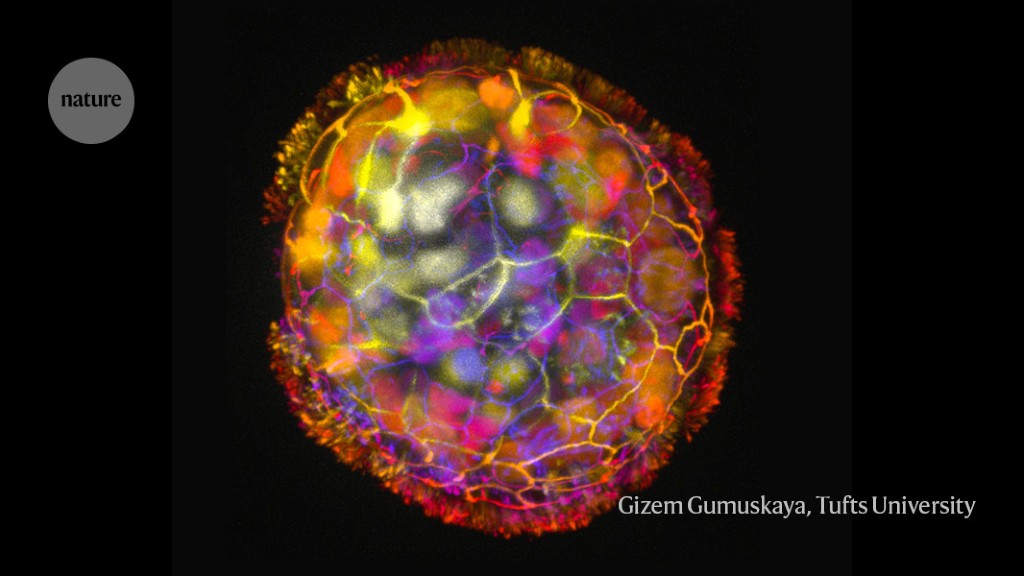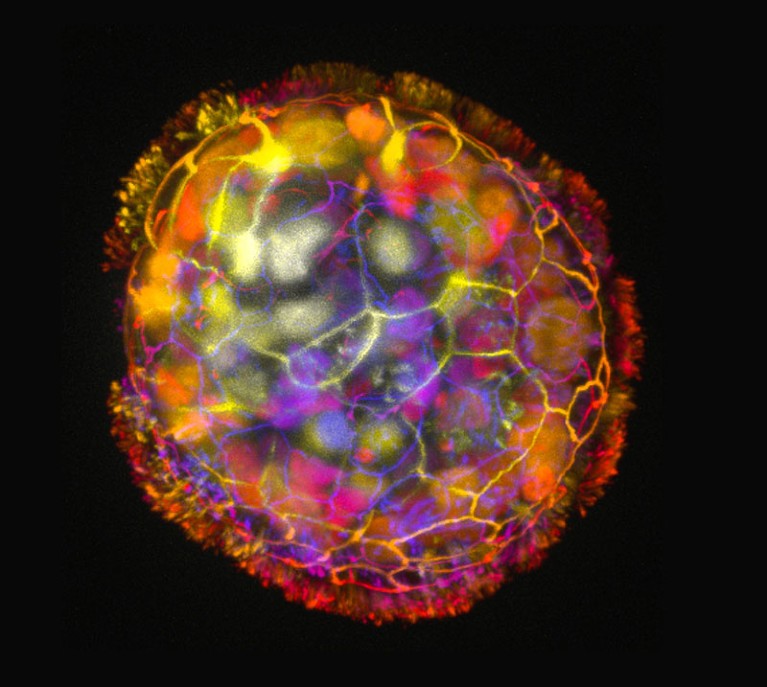
Each anthrobot is made up of a few hundred cells.Credit: Gizem Gumuskaya, Tufts University
Scientists have recently developed anthrobots composed of human cells, designed to repair damaged neural tissue1. Aptly named ‘anthrobots’, they are created from human tracheal cells and can potentially revolutionize personalized medicine.
This cutting-edge research may pave the way for a new field of ‘tissue engineering 2.0’, offering synthetic control over various developmental processes, according to Alex Hughes, a bioengineer at the University of Pennsylvania in Philadelphia.
Although developmental biologist Michael Levin and his team at Tufts University had initially developed similar robots using embryonic frog cells, it was limited in its medical applications. The team’s latest innovation, the anthrobots, are self-assembling and are currently under investigation for their potential therapeutic uses with human tissue grown in a laboratory. Advanced Science published their remarkable findings.
The process of creating these anthrobots involved growing clusters of human tracheal skin cells, allowing tiny hairs called cilia to move to the outside of the spheroids. The result is anthrobots, each consisting of a few hundred cells, displaying various patterns of movement. Remarkably, these anthrobots can merge to form a ‘superbot’, capable of healing damaged neural tissue.
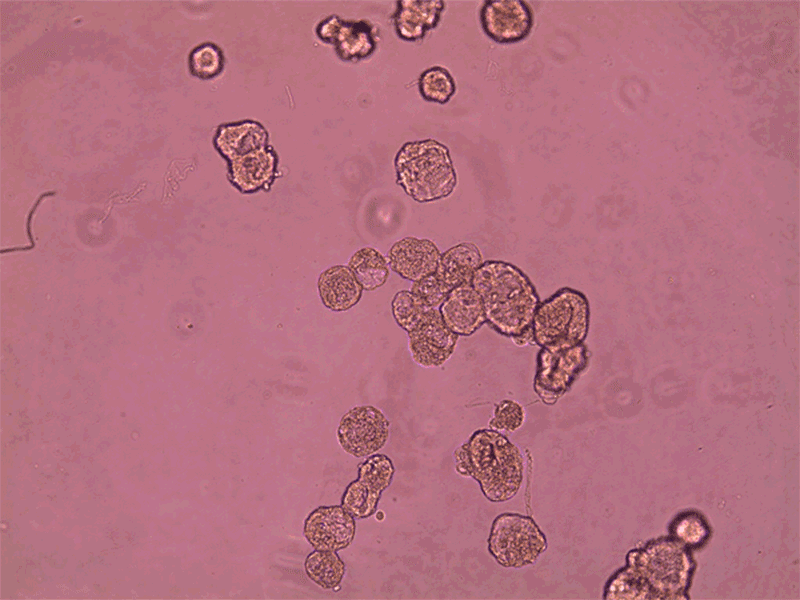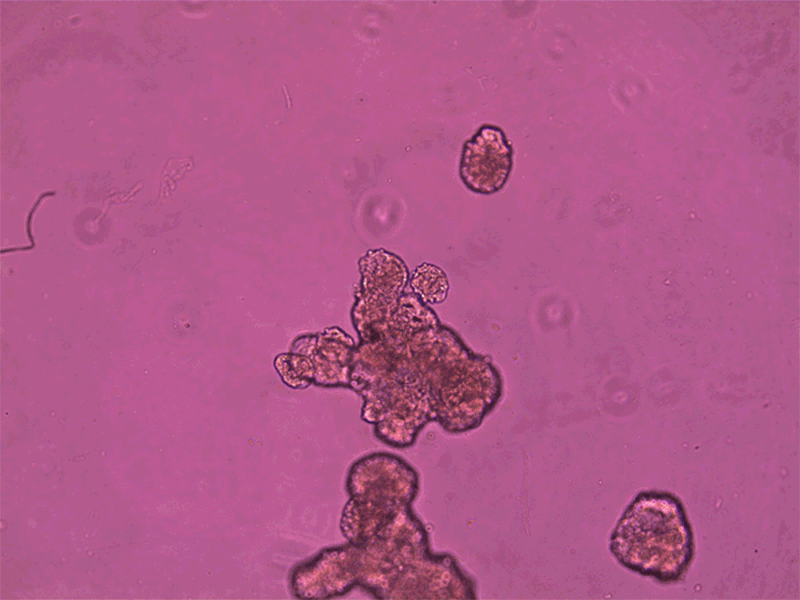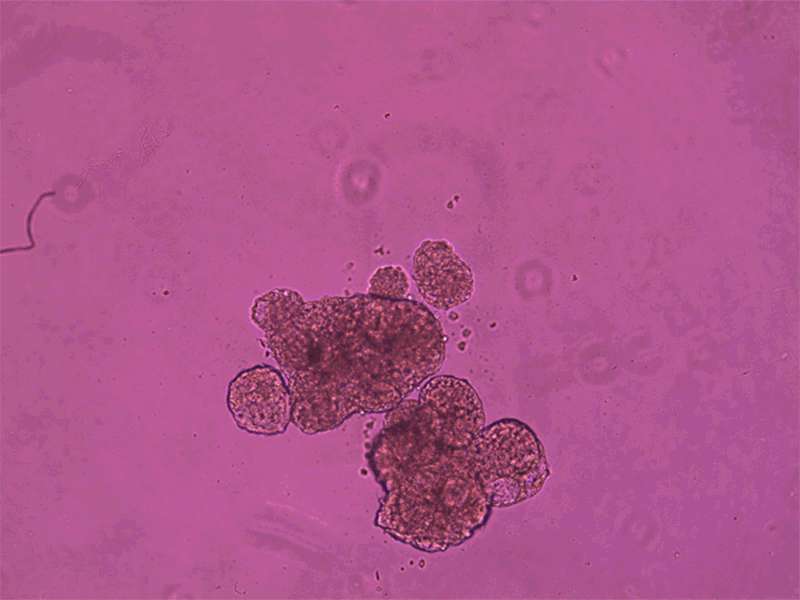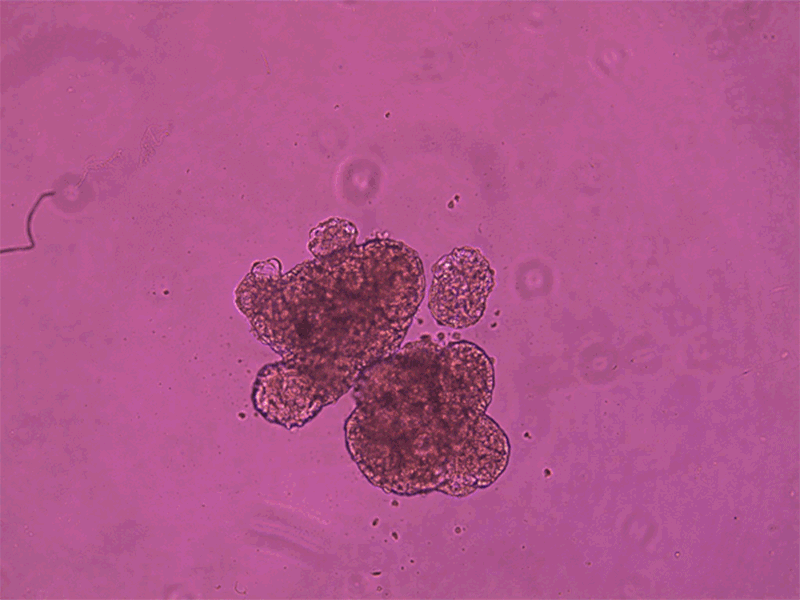
Anthrobots can spontaneously fuse together to form a larger structure called a superbot, which was able to encourage the growth of neurons (not shown).Credit: Gizem Gumuskaya, Tufts University
In addition to their potential for repairing neural tissue, anthrobots made from a person’s own tissue could potentially be used for various medical applications such as clearing arteries, breaking up mucus, and delivering drugs. By combining different cell types and exploring other stimuli, it may even be possible to develop biobots — robots made from biological material — with applications in fields such as sustainable construction and outer-space exploration.
“Once we understand what cell collectives are willing and able to do, then we can begin to control that not just for stand-alone bots, but for regenerative medicine,” says Levin, emphasizing the potential for regrowing limbs and other medical breakthroughs.