The recent full FDA approval of Lecanemab, also known as Leqembi, marks a significant milestone in the treatment of Alzheimer’s disease. This groundbreaking drug works by reducing amyloid plaques in the brain, a key characteristic of the disease. In clinical trials, it has shown promising results, slowing cognitive decline by 27%.
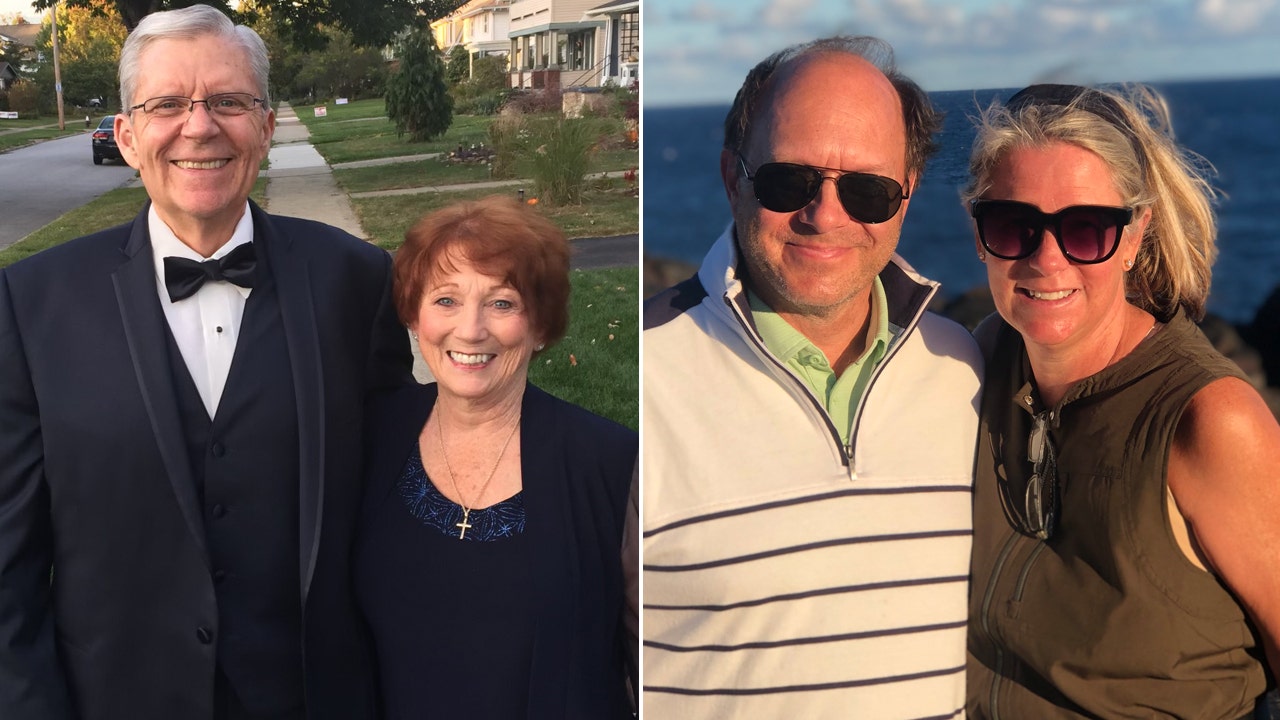
Fox News Digital recently interviewed two Ohio patients who participated in the trials and shared their personal experiences with Leqembi. Joan Murtaugh, a 77-year-old from Lakewood, Ohio, first noticed memory issues seven years ago and was later diagnosed with mild cognitive impairment. She participated in the Phase 3 trial of Leqembi at Cleveland Clinic in 2020, receiving regular infusions of the drug. Although she is unsure if she received the real drug or a placebo, she has experienced stable symptoms and potentially even some improvement. Throughout the trial, she remains fully functional and continues to engage in daily activities. Murtaugh finds hope in this new drug, describing it as a ray of sunshine amidst the darkness of Alzheimer’s.
Another patient, retired attorney John Domeck, was diagnosed with Alzheimer’s at the young age of 57. Domeck and his wife, Ann, noticed his memory lapses, leading to a diagnosis in 2019. Domeck participated in a clinical trial for Leqembi for 18 months, not knowing whether he received the drug or placebo. After being switched to the open-label medication, he has maintained a stable condition for the past four years. Domeck’s cognitive deficits have only minimally increased, which is promising given the progression of the disease. He continues to lead an active life, participating in activities like golfing and reading. The support and care from their Cleveland Clinic team have left the Domecks astounded and grateful for their experience with Leqembi.
While Leqembi is not a cure for Alzheimer’s, it represents a significant advancement in targeting the underlying disease rather than just managing symptoms. The approval of this drug signifies a new era of treatment options for Alzheimer’s patients.
Denial of responsibility! VigourTimes is an automatic aggregator of Global media. In each content, the hyperlink to the primary source is specified. All trademarks belong to their rightful owners, and all materials to their authors. For any complaint, please reach us at – [email protected]. We will take necessary action within 24 hours.